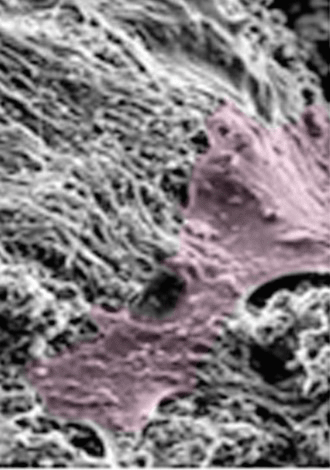
Of the 5.6 million bone fractures that occur annually in the United States, about 10% fail to repair. These patients then need to undergo surgery for the bone to heal completely. Two common treatments are implants and autologous bone grafting which can be costly, ineffective, and painful. Therefore, recently there has been an interest in using human mesenchymal stem cells (hMSCs) because of their ability to promote bone healing. Studies have shown that hMSCs are not retained at the injection site for a sufficient amount of time to effectively promote bone repair. There is therefore a clear need for self-sustaining, biocompatible implants that reflect the osteogenic niche and interact with surrounding cells to promote bone regeneration.
We have demonstrated that inhibiting peroxisome proliferator activated receptor-γ (PPAR-γ) with the small molecule GW9662 promotes the establishment of a pro-osteogenic hMSC phenotype (OEhMSCs). The OEhMSCs secrete extracellular matrix that mimics the composition of regenerating bone tissue (hMatrix). hMatrix coadministered with OEhMSCs dramatically enhances retention of osteogenic stem cells and results in rapid repair of bone defects in mice. We integrating these concepts to develop composite microspheres for coadministration of hMatrix, GW9662 and hMSCs into bone defects and quantitatively assess bone repair and pro-osteogenic soluble factor secretion. Ultimately, this work is aimed at providing an injectable vehicle for delivering hMSCs to bone defects to promote bone regeneration.
Funding: National Institute of Arthritis and Muscloskeletal Diseases and National Science Foundation
Collaborators: Drs. Carl Gregory and Allison Rice-Ficht (Texas A&M Health Science Center) and Dr. Jun Kameoka (Texas A&M University)
Studying Bone Tumor and Host Tissue Interactions Using Micro-Gravity Bioreactors
 Osteolytic
bone tumors result in catastophic tissue damage, resulting in crippling
pain, loss of bone strength, and a supportive stroma for tumor cells.
DKK-1 is a soluble Wnt inhibitor implicated in some osteolytic cancers.
Using rotating wall bioreactors to simlate microgravity on earth,
OEhMSCs and bone tumor cells expressing DKK-1 are
co-cultured on microspheres coated with hMatrix to study the
ability of DKK-1 to inhibit OEhMSC differentiation into an osteoblastic
phenotype. These experiments are scheduled for translation to the
International Space Station in the coming year. THe ultimate goal is to
develop a disease model for identifying therapeutics to reverse bone
destruction caused by osteolytic tumors.
Osteolytic
bone tumors result in catastophic tissue damage, resulting in crippling
pain, loss of bone strength, and a supportive stroma for tumor cells.
DKK-1 is a soluble Wnt inhibitor implicated in some osteolytic cancers.
Using rotating wall bioreactors to simlate microgravity on earth,
OEhMSCs and bone tumor cells expressing DKK-1 are
co-cultured on microspheres coated with hMatrix to study the
ability of DKK-1 to inhibit OEhMSC differentiation into an osteoblastic
phenotype. These experiments are scheduled for translation to the
International Space Station in the coming year. THe ultimate goal is to
develop a disease model for identifying therapeutics to reverse bone
destruction caused by osteolytic tumors.Funding: Center for the Advancement of Science in Space
Collaborators:Dr. Carl Gregory (Texas A&M Health Science Center) and Dr. Jun Kameoka (Texas A&M University:
Cytoskeletal Adaptation to Applied Forces

Most animal cells generate intracellular forces that are transmitted to, and countered by, forces in the extracellular matrix. This mechanical force balance is necessary for maintaining both mechanical and biochemical cell homeostasis. When this balance is disturbed, such as when the matrix is cyclically stretched, the cell cytoskeleton reorganizes in an attempt to reestablish homeostasis. For example, arterial endothelial cells (ECs), which are elongated and aligned with the vessel axis in most of the arterial tree, lack such alignment at regions prone to atherosclerosis. We have shown that cyclic stretching of ECs induces activation of mitogen-actived kinases (MAPKs), a signaling protein involved in regulating pro-atherogenic gene expression, but that MAPK activations subside as cells and their stress fibers align perpendicular to stretch. Other studies, both in vitro and in vivo, support a relationship between cell alignment and an anti atherogenic EC phenotype, yet the mechanism remains obscure. We have developed a theoretical model based on actomyosin cross-bridge cycling to describe the dynamic relationships between deformations in the matrix and associated reactive reorganization and force dissipation of the actin cytoskeleton. This model predicts that MAPK activity correlates with stress fiber tension. Our most recent studies demonstrate that the direction of stretch-induced human mesenchymal stem cell alignment strongly depends on matrix mechanical properties.
Funding: National Science Foundation and American Heart Association
Collaborator: Dr. Shinji Deguchi (Nagoya Institute of Technology)
Fluid Shear-Induced Sprouting Angiogenesis
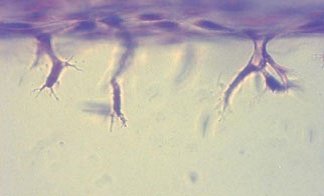
The process of sprouting angiogenesis involves activating endothelial cells in a quiescent monolayer of an existing vessel to degrade and migrate into the underlying extracellular matrix to form new blood vessels. Sprouting primarily occurs in the postcapillary venules, where WSS is estimated to range from 1 to 8 dyn/cm2. While the roles of biochemical factors in angiogenic sprouting have been well characterized, the roles of fluid forces have received much less attention. Using a model of endothelial invasion into three-dimensional collagen matrix developed by our collaborator, Prof. Kayla Bayless, we have demonstrated that fluid shear stress upregulates invasion. Interestingly, maximal invasion occurs at a shear stress magnitude of ~5 dyn/cm2. Our studies show that pro-angiogenic signaling molecules (Akt) and matrix proteases (MMP2 and MT1-MMP) are also maximally upregulated at this level of shear stress, demonstrating molecular mechanisms that regulate invasion induced by fluid forces.
Funding: National Heart, Lung and Blood Institute
Collaborators: Dr. Kayla Bayless (Texas A&M Health Science Center) and Dr. Alvin Yeh (Texas A&M University)